OBJECTIVES: After having completed this unit, students should be able to:
- Define titration, titration curve, indicator, end-point, equivalence point and mid point (half-equivalence point).
- Sketch a titration curve and identify various points on the curve.
- Calculate the pH at various points along a titration curve for a strong acid with a strong base, strong base with a strong acid, weak acid with strong base or weak base with strong acid given the volume of the titrant and the concentration of the components.
- Calculate the Ka for a weak acid or Kb for a weak base from titration curve data.
Reading and Homework Exercises
Table of Contents from: OpenStax Chemistry 2e: Section 14.7
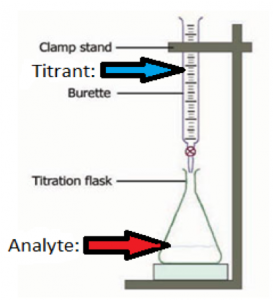
14.7 Acid-Base Titrations
Practice
Question 1:
Question 2:
Question 3:
Question 4:
Question 5:
Question 6:
Question 7:
Question 8:
Question 9:
Question 10:
Question 11: