This is a 9 point assignment.
OBJECTIVES: After having completed this unit, students should be able to:
- Define titration, titration curve, indicator, end-point, equivalence point and mid point (half-equivalence point).
- Sketch a titration curve and identify various points on the curve.
- Calculate the pH at various points along a titration curve for a strong acid with a strong base, strong base with a strong acid, weak acid with strong base or weak base with strong acid given the volume of the titrant and the concentration of the components.
- Calculate the Ka for a weak acid or Kb for a weak base from titration curve data.
Reading and Homework Exercises
Table of Contents from: OpenStax Chemistry 2e: Section 14.7
14.7 Acid-Base Titrations
Questions 1-5 address general understanding of acid-base titration curves
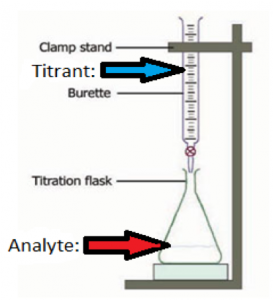
Question 1:
Question 2:
Question 3:
Question 4:
Question 5:
Questions 6-9 cover pH calculations at various points of four titrations (not necessarily in the order below):
- strong acid with strong base
- weak acid with strong base
- strong base with strong acid
- weak base with strong acid.
Before starting each problem, you first need to recognize which type of titration it is.
Question 6:
Question 7:
Question 8:
Question 9:
Questions 10 and 11 cover other types of titration questions you might encounter
Question 10:
Question 11: