This is a longer assignment than your previous ones, due to the fact that this is a longer topic than the Unit 1 topics and that there are only two topics in Unit 2. As always, if you encounter questions you cannot answer or don’t understand, follow up with the Learning Commons tutors and/or your instructor.
This is a 20 point assignment.
OBJECTIVES: After having completed this unit, students should be able to:
- Manipulate any mathematical equation involving logarithms.
- Define or explain and apply the definition for the following terms: reaction rate, average and instantaneous rates, rate constant, reaction order, rate law, half-life, activated complex, activation energy, mechanism, elementary reactions (elementary steps), molecularity, rate determining step, catalyst (homogeneous and heterogeneous)
- Distinguish among the average, instantaneous and initial rates for a chemical reaction.
- Explain the effect of the physical state of the reactants, frequency, kinetic energy (temperature), catalyst, and orientation of collisions on the reaction rate.
- Express the rate of a reaction in terms of the change in concentration of all reactants and products given the balanced chemical equation for the reaction.
- Calculate the rate of formation or consumption for a reactant or product in a chemical reaction given the rate of formation or consumption any other reactant or product in the reaction.
- Determine the order of a reaction, rate law and calculate the rate constant k (and the units of k) for a reaction from a series of experiments given the measured initial rates for various concentrations of reactants.
- Using the integrated rate law equation for first order reactions only, calculate the rate constant, half-life, time or percent of starting material given any two of the variables.
- Determine whether a rate law for a reaction of form A → products is zero, 1st, or 2ndorder given concentration vs. time data. Know what graphs can be used to make this determination, and what quantities are represented but the slope of the resulting line.
- Determine the rate constant from the slope and the concentration at a given time from the integrated rate law equation resulting from the plot of first-order data.
- Summarize the two types of rate laws and the methods by which they can be determined.
- Write the rate law for elementary reactions/steps.
- Discuss the relationship between the reaction mechanism and the rate law.
- Determine the molecularity of each elementary reaction, rate determining step and the overall balanced equation given the mechanism for a reaction.
- Given the mechanism for a reaction write the rate law.
- Given a mechanism in which the rate determining step is not the first step, write the rate law.
- Describe the collision model for chemical reactions.
- Draw reaction profile diagrams and identify reactants, products, the activated complex, Eactand ΔE.
- Describe the effect of a catalyst on the energy profile diagram of a reaction.
- Explain how a catalyst works and how it fits into the collision model for chemical reactions.
- Identify the catalyst and the intermediate given the mechanism for a reaction.
- Explain the temperature dependence of reaction rates.
- Define and calculate Eact, the Arrhenius constant or frequency factor (A), rate constant (k), or temperature for a chemical reaction.
- Using two sets of temperature and rate constants, calculate any of the following variables: the activation energy, the temperature, or rate constant
Reading and Homework Exercises
Table of Contents from: OpenStax Chemistry 2e: Chapter 12
Questions 1-3 allow you to check your understanding of the concept of reaction rates and understanding of how the rates of consumption and production of various reactants and products in a reaction are related.
Introduction
12.1 Chemical Reaction Rates
Questions 1-3 allow you to check your understanding of the concept of reaction rates and understanding of how the rates of consumption and production of various reactants and products in a reaction are related.
Question 1:
Question 2:
Question 3:
12.2 Factors Affecting Reaction Rates
Read this section now and keep these factors in mind. Reading and homework exercises in the remaining sections will demonstrate why these factors affect rates the way they do.
12.3 Rate Laws
Questions 4-12 check your understanding of the various parts of rate laws and your ability to write a correct rate law given data.
Question 4:
Question 5:
Question 6:
Question 7:
Question 8:
The next four questions use the data table below:
Table 1
| [A] | [B] | Initial rate of production of C |
| M | M | M/s |
| 0.200 | 0.0800 | 0.500 |
| 0.200 | 0.160 | 1.00 |
| 0.400 | 0.160 | 4.00 |
Question 9:
Question 10:
Question 11:
Question 12:
12.4 Integrated Rate Laws
Questions 13-21 check your understanding of integrated rate laws for zero, first, and second order reactions and their graphs. Also included are calculations using integrated rate laws (first order only).
Question 13:
Question 14:
Question 15:
Question 16:
Question 17:
Question 18:
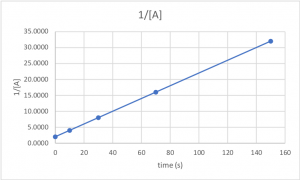
The next three questions use the data from Table 2, which is plotted in Figures 1,2,and 3.
A chemical reaction in which the reactant A is decomposed into products is carried out. The concentration of A is measured as a function of time (data in Table 2). The ln[A] and 1/[A] data were calculated from the concentrations and are also in Table 2.
Table 2
Figures 1-3 are plots of the data in Table 2.
| time (s) | [A] | ln[A] | 1/[A] |
| 0 | 0.50000 | -0.69315 | 2.0000 |
| 10 | 0.25000 | -1.38629 | 4.0000 |
| 30 | 0.12500 | -2.07944 | 8.0000 |
| 70 | 0.06250 | -2.77259 | 16.0000 |
| 150 | 0.03125 | -3.46574 | 32.0000 |
Figure 1. [A] vs. time
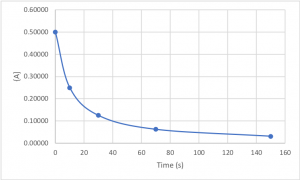
Figure 2. ln[A] vs. time
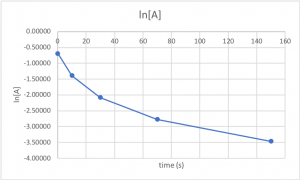
Figure 3. 1/[A] vs. time
Question 19:
Question 20:
Question 21:
12.5 Collision Theory
The next two questions use the reaction profile diagram shown in Figure 4.
Figure 4
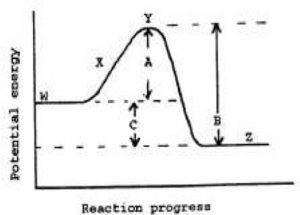
Question 22:
Question 23:
Questions 24-26 test your understanding of activation energy and Arrhenius Law calculations and effects of various factors on reaction rate.
Question 24:
Question 25:
Question 26:
12.6 Reaction Mechanisms
Questions 27-36 check your understanding of reaction mechanisms and their relationship to concepts learned earlier in your study of chemical kinetics.
Consider the following proposed reaction mechanism:
Step 1: F2 + 2 NO2 → NO2F + F + NO2
Step 2: F + NO2 → NO2F
The first step is the slow (rate-determining) step. Answer the following questions:
Question 27:
Question 28:
Question 29:
Question 30:
Question 31:
Question 32:
Consider the following proposed reaction mechanism:
Step 1: NO + NO → N2O2
Step 2: N2O2 + H2 → N2O + H2O
Step 3: N2O + H2→N2 + H2O
The second step is the slow (rate-determining) step. Answer the following questions:
Question 33:
Question 34:
Question 35:
Question 36:
12.7 Catalysis
The final two questions use the reaction profile diagram shown in Figure 5.
Figure 5
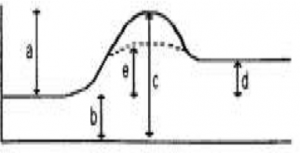
Question 37:
Question 38: